The recent direct functionalization of transition metal-catalyzed inert carbon-hydrogen bonds has received great attention from chemical researchers and has made important progress. In such reactions, severe reaction conditions, the use of equivalent oxidants, and the difficulty in controlling selectivity are still major constraints in their application. In addition, the work of olefin hydrocarbon-hydrogen bond activation from olefins is also very rare.
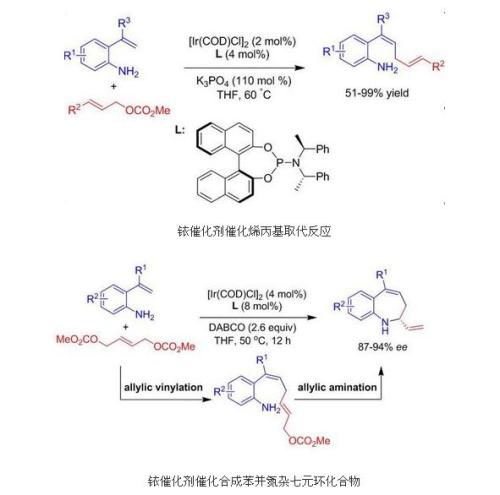
In 2009, researchers from the State Key Laboratory of Organometallics of the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, discovered that metal ruthenium catalysis was based on free amine-assisted double-bonded terminal hydrogen-carbon bond activation in [Ir(COD)Cl]2 and Feringa Under the catalytic system of body, allyl styrene compounds and allyl carbonate can undergo direct allyl acetylation reaction, and cis-double bond products are obtained stereoselectively (J. Am. Chem. Soc 2009, 131, 8346-8346.) The reaction conditions are mild and the raw materials are readily available. This method provides new strategies and ideas for constructing cis double bonds. After the publication of the results, they were actively reviewed by Synfacts (Synfacts, 2009, 9, 0987). This is also the first case of a metal ruthenium-catalyzed direct allyl acetylation reaction.
Recently, the researchers based on this discovery, through the clever design, in the [Ir (COD) Cl] 2 and Feringa ligand catalysis, o-amino styrenite and allyl methyl dicarbonate reaction ,Allyl alkenylation can be achieved in series with the asymmetric amination of allyl amines, high yield, high enantioselective synthesis of benzo aza 7-membered ring compounds. The obtained optically active benzoazepine seven-membered ring compounds can be conveniently converted into complex multicyclic compounds, which are widely used in many natural products and drug molecules for the synthesis of benzo aza 7-membered rings. A type of skeleton provides an effective method. This part of the work has been published in Angew. Chem. Int. Ed., 2010, 49, 1496-1499. After the results were published, they were again actively reviewed by Synfacts (Synfacts, 2010, 4, 0446).
These researches were funded by the National Natural Science Commission and the 973 project of the Ministry of Science and Technology.