Electrochemical double-layer capacitors, also known as supercapacitors, store energy through the reversible absorption and desorption of electrolyte ions on the surface of high surface area electrodes. Since no charge transfer kinetic limitations such as redox reactions are involved, supercapacitors can operate at extremely high charge and discharge rates and have a good cycling capacity of up to a million times, making them widely used in the field of energy storage. Graphene can theoretically have a specific capacity of 550 F / g, which has attracted much attention as an electrode material for supercapacitors. However, the performance of graphene-based materials is still far below expectations. On the one hand, the quantum capacitance of graphene has been shown to play a key role in the establishment of electric double layer capacitance; on the other hand, interface electrochemistry is a key factor in determining the energy storage performance of supercapacitors, which involves the transmission of ions in the electrode channels Diffusion, adsorption / desorption of ions on the carbon surface, etc. The dynamic charge separation mechanism at the graphene-electrolyte interface is still not well resolved, hindering the further development of high-performance 2D or 3D graphene electrodes.
Recently, the research group of Zhu Yanwu, University of Science and Technology of China, proposed that a single layer of graphene with low defect content can provide an excellent template for understanding the ion adsorption / interaction at the graphene interface under polarization: both eliminating the channel ion limitation effect, It is not affected by pores or defects in most porous carbon materials (National Science Review, 2019). Based on this, the research group and the French Patrice Simon research group used electrochemical impedance spectroscopy and an electrochemical quartz crystal microbalance system to study in situ the dynamics of ionic liquid (EMI-TFSI) electrolyte on the surface of single-layer graphene response. The study found that in the anodized interval of graphene, charge storage is dominated by desorption of positively charged cluster ions; in the anodized interval, the surface mass of graphene changes little, showing the effect of surface ion rearrangement. As the applied potential increases, the two types of interface response dominate the changes in the electric double layer, resulting in an increase in the electric double layer capacitance. This study provides a basis for further understanding of the graphene-electrolyte interface structure and the energy storage of the graphene electric double layer.
The research results were published in the Journal of the American Chemical Society under the title of Charge Storage Mechanisms of Single Layer Graphene in Ionic Liquid. The research work was supported by the Natural Science Foundation of China and the National Study Fund (CSC) project.
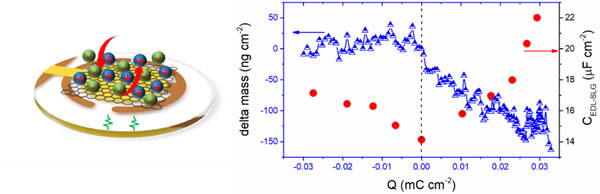
Schematic diagram of quartz microcrystalline balance used for in-situ electrochemical detection (left), observed ion response of graphene surface (right)
Ratchet Tie Down,Ratchet Straps,Small Ratchet Straps,Best Ratchet Straps
Jiangsu Zhongyi Work Rigging Co., Ltd. , https://www.zy-rigging.com