 |
In the non-cubic chalcopyrite structural compounds, the “èµcubic†structure design method achieves a highly degenerate energy band similar to the cubic structure, resulting in good electrical transport performance and thermoelectric performance. (a) Schematic diagram of the crystal structure and energy band structure of the cubic sphalerite structure. (b) Schematic representation of the crystal structure and energy band structure of a ternary chalcopyrite structure compound. The "cubic" structure consists of a cubic cation framework and a non-cubic twisted anionic lattice. (c) The relationship between the ZT value and the splitting value ΔCF of the valence band top energy band. The red dots represent the reported high temperature compounds with ZT>1, and the blue and green dots represent (Ag,Cu)InTe2 and Cu(In,Ga)Te2 solid solutions, respectively.
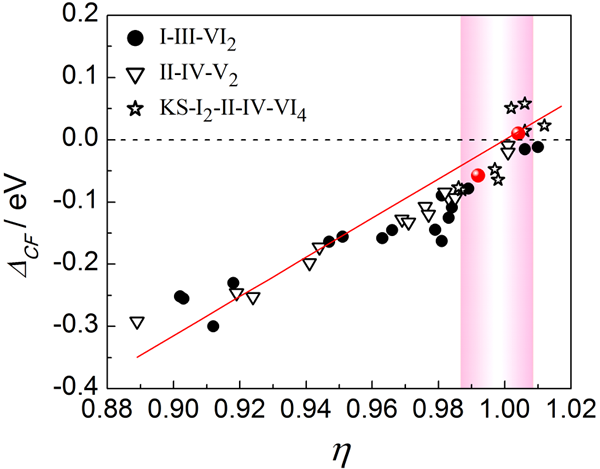
Figure 2 Unity-η screening criteria. The red dots represent the reported high temperature compounds with ZT >1. The pink band marks the η≈1 area (a possible area where thermoelectric compounds may exist).
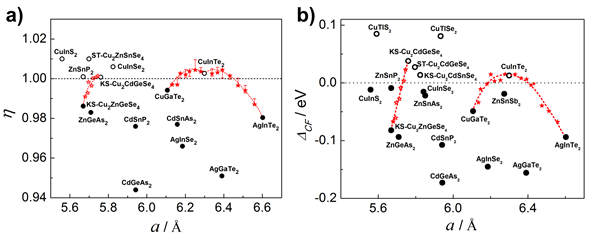
Figure 3 (a) Design of η vs. a and (b) ΔCF vs. a solid solution compounds, where the red star represents two compounds with η < 1 (ΔCF <0) and η> 1 (ΔCF>0). The resulting solid solution, the red dotted line is the trend line.
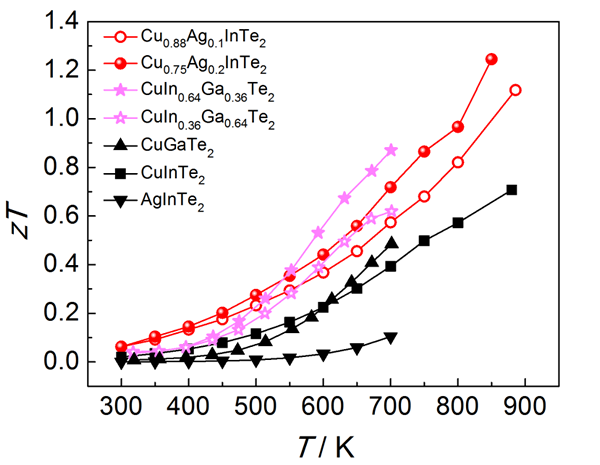
Fig. 4 Thermoelectric value of CuInTe2 and AgInTe2 (CuGaTe2) and their solid solutions
Developing industrial applications of high-performance thermoelectric conversion materials and expanding thermoelectric conversion technology is the primary task in the field of thermoelectric science and technology. The performance of thermoelectric materials can generally be measured by a dimensionless composite index (thermal electric potential value, ZT), depending on the material's intrinsic physical properties (Seebeck coefficient, conductivity, thermal conductivity, and absolute temperature). For a long time, the scientific research of thermoelectric materials has mainly concentrated on limited material systems such as SiGe, PbTe, skutterudite, Half-Heusler, Mg2Si, and Cu2Se.
Most of these thermoelectric compounds have a highly symmetrical cubic structure. The highly symmetrical crystal structure usually has a highly degenerate or multi-energy valley structure at the top of the valence band or at the bottom of the conduction band, and thus has good electrical transport properties. In nature, compounds mainly consist of non-cubic structures. There are many narrow bandgap and low thermal conductivity materials. However, these low-symmetry non-cubic semiconductor compounds have been ignored by thermoelectric researchers. How to screen out new thermoelectric materials from numerous non-cubic structural compounds has become a huge challenge for thermoelectric materials research.
The diamond-like structure compound is derived from a diamond structure and consists of a tetrahedron composed of a metal or semiconductor element and a carbon-like SP3 hybrid bond. Due to the different atomic radii and chemical valences of the constituent elements, the lattice of the material is distorted from the cubic structure of the diamond to a non-cubic structure. At the same time, the lattice distortion can easily cause intrinsic defects in the crystal lattice, resulting in thermal conduction. The rate is low.
When the type and atomic ratio of the constituent elements are changed, the band gap of the material is also changed, and the value thereof covers a narrow band semiconductor of 1 eV or less and a wide band gap semiconductor or insulator of several eV. The intrinsically low thermal conductivity and tunable electrical properties of diamond-like structural compounds make them promising as excellent thermoelectric materials.
The Shanghai Institute of Ceramics, Chinese Academy of Sciences, previously reported that the thermoelectric value of CuInTe2, a tetragonal quasi-diamond structural compound, reached 1.18 at 850K (Chem. Commun. 2012, 48, 3818.). Later Japanese scholars reported the thermoelectricity of CuGaTe2 with the same tetragonal structure. The merit value is up to 1.4 (Adv. Mater. 2012, 24, 3622.), so that the thermoelectric properties of the diamond-like structure compound can be compared with that of a conventional thermoelectric material. However, the mechanism of high thermoelectric properties in non-cubic diamond-like compounds is not clear, limiting the exploration and design of many other compounds with similar non-cubic structures.
Recently, Zhang Wenqing, Shi Xun, and Chen Lidong, researchers of the Shanghai Institute of Ceramics, Chinese Academy of Sciences, collaborated with Professor Jihui Yang of the University of Washington, and Professor Ctirad Uher of the University of Michigan to use non-cubic chalcopyrite (tetragonal) diamond-like compounds as an example. Based on the theoretical calculation of the material genome engineering and combined with experiments, the idea of ​​the â€œèµ cubic†microstructure was proposed to screen and design novel thermoelectric compounds with non-cubic structures.
The â€œèµ cubic†microscopic structural design idea (Figure 1) refers to the fact that some long-range ordered ionic lattices form a cubic or near-cubic framework to achieve energy band convergence and improve electrical transport performance; other partially distorted ion lattices are Short tetrahedral irregular tetrahedrons with different bond lengths, bond angles, and arrangements are formed to block heat transfer and reduce lattice thermal conductivity. The â€œèµ cubic†micro-structure design idea can realize the coordinated regulation of electric transport and heat transport, so a high thermoelectric figure of merit can be obtained.
The study found that the η = 1 (Unity-η) screening rule for realizing the high thermoelectric performance of this type of material (Fig. 2), that is, in the tetragonal diamond-like compound, when the square variable parameter η (=c/2a) is close to 1, The valence splitting value ΔCF of the valence band in the electronic structure is close to 0 (Fig. 2). At this time, the cations form a long-range or cubic cubical grid, while the anions form a non-cubic grid with a short-range disorder. Convergence or overlap occurs with optimal electrical transport performance and thermoelectric value.
Previously reported high-performance thermoelectric materials CuInTe2 and CuGaTe2 η value is almost 1, fully consistent with our simple screening rules. Utilizing the Unity-η rule, potentially thermoelectric compounds that may have excellent performance can be screened out from dozens of chalcopyrite tetragonal compounds.
For many diamond-like structural compounds with η not near 1, a design principle for solid solution compounds is proposed (Fig. 3), that is, η < 1 (ΔCF <0) and η> 1 (ΔCF> 0), and the lattice mismatch is not Large two or more compounds form a solid solution, and η≈1 (ΔCF≈0) is achieved by adjusting the solid solubility to find suitable components, thereby obtaining good electrical transport and thermoelectric properties. Based on the η(ΔCF) vs. a solid solution map, three solid solutions were designed (red in Figure 3). CuInTe2 and AgInTe2 solid solutions were verified by our experiments, and the thermoelectric figure of merit was significantly higher than that of the matrix (Figure 4). The “èµcube†microstructure design idea provides new research ideas and guidance tools for exploring non-cubic structural material systems with high thermoelectric performance.
For the Peristaltic Pump Tubing, We have different material choices for your various liquids. The standard tube is silicon tubing which conforms to the FDA certificate. Except that we also offer the Saint-Gobain imported tubing, Eg.: Norprene®A-60-F/Norprene®chemical /Pharmed® BPT... Which suitable for various chemical-based liquid.
Suitable for various industrial applications.
Norprene Chemical Tube,Peristaltic Pump Transfer Tube,Peristaltic Pump Hose,Peristaltic Pump Tube
Baoding Chuangrui Precision Pump Co., Ltd. , https://www.crperistalticpump.com